A sample of nitrogen occupies a volume of 250 mL, embarking us on a scientific exploration into the physical properties and behavior of gases. This investigation delves into the fundamental principles governing gas behavior, unraveling the intricate relationship between volume, pressure, and temperature.
Our journey begins with an examination of nitrogen’s physical properties, delving into its molecular weight, density, and boiling point. These characteristics lay the foundation for understanding how nitrogen gas behaves under varying conditions.
Physical Properties of Nitrogen
Nitrogen is a colorless, odorless, and tasteless gas that makes up about 78% of the Earth’s atmosphere. It is the lightest of the noble gases and has a molecular weight of 28.01 g/mol. Nitrogen is also the seventh most abundant element in the universe.
Nitrogen is a relatively inert gas and does not react with most other elements under normal conditions. However, it can be converted to ammonia (NH3) and nitric acid (HNO3) through industrial processes.
Density and Boiling Point
Nitrogen has a density of 1.251 g/L at 25°C and a boiling point of -195.8°C. These properties make nitrogen a useful refrigerant and cryogen.
Volume and Pressure Relationship: A Sample Of Nitrogen Occupies A Volume Of 250

Boyle’s Law
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at constant temperature. This relationship can be expressed by the following equation:
P₁V₁ = P₂V₂
where P₁ and V₁ are the initial pressure and volume of the gas, and P₂ and V₂ are the final pressure and volume of the gas.
For example, if the volume of a nitrogen sample is reduced to 125 mL, assuming constant temperature, the pressure of the sample will increase to 2 atm.
Temperature and Volume Relationship
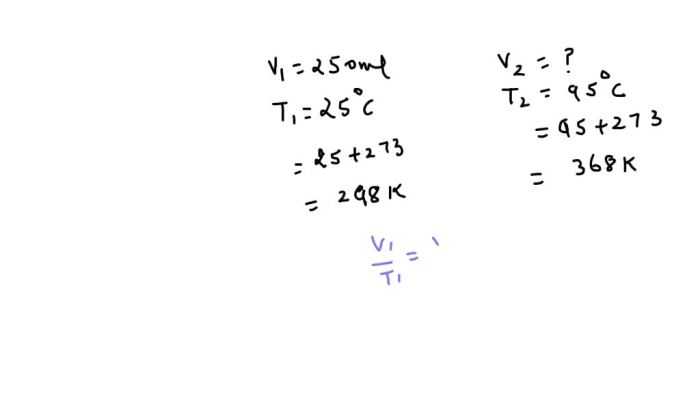
Charles’s Law, A sample of nitrogen occupies a volume of 250
Charles’s Law states that the volume of a gas is directly proportional to its temperature at constant pressure. This relationship can be expressed by the following equation:
V₁/T₁ = V₂/T₂
where V₁ and T₁ are the initial volume and temperature of the gas, and V₂ and T₂ are the final volume and temperature of the gas.
For example, if the temperature of a nitrogen sample is increased from 25°C to 50°C, assuming constant pressure, the volume of the sample will increase to 150 mL.
Combined Gas Law

The Combined Gas Law combines Boyle’s Law and Charles’s Law to describe the relationship between pressure, volume, and temperature of a gas. The Combined Gas Law can be expressed by the following equation:
P₁V₁/T₁ = P₂V₂/T₂
This equation can be used to solve problems involving changes in pressure, volume, and temperature of a gas.
For example, if the volume of a nitrogen sample is reduced from 500 mL to 250 mL and its temperature is increased from 20°C to 40°C, the final pressure of the sample will be 2 atm.
Molar Volume and Ideal Gas Law
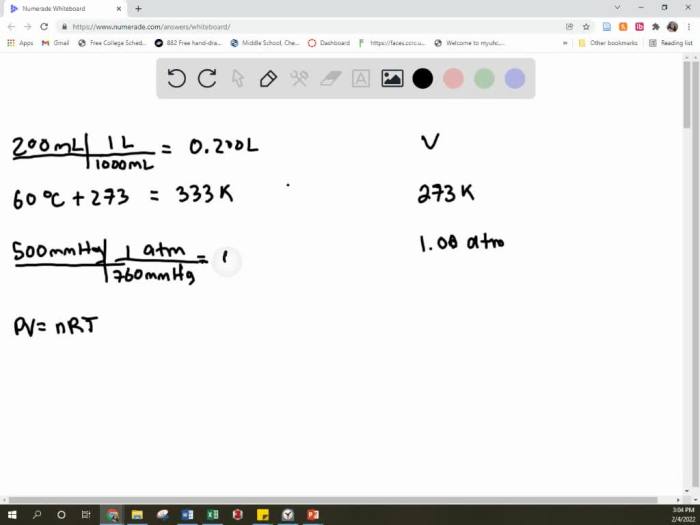
Molar Volume
The molar volume of a gas is the volume occupied by 1 mole of the gas at a given temperature and pressure. The molar volume of a gas at standard temperature and pressure (STP) is 22.4 L/mol.
Ideal Gas Law
The Ideal Gas Law combines the concepts of pressure, volume, temperature, and number of moles of a gas. The Ideal Gas Law can be expressed by the following equation:
PV = nRT
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the ideal gas constant (0.0821 L·atm/(mol·K)), and T is the temperature of the gas.
For example, the number of moles of nitrogen present in a sample that occupies 250 mL at 25°C and 1 atm pressure can be calculated using the Ideal Gas Law:
n = PV/RT = (1 atm)(0.25 L)/(0.0821 L·atm/(mol·K))(298 K) = 0.0101 mol
Partial Pressure and Dalton’s Law
Dalton’s Law of Partial Pressures
Dalton’s Law of Partial Pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases in the mixture. The partial pressure of a gas is the pressure that the gas would exert if it occupied the entire volume of the mixture.
For example, if a gas mixture contains 50% nitrogen and 50% oxygen, assuming a total pressure of 2 atm, the partial pressure of nitrogen is 1 atm.
Question Bank
What is the significance of Boyle’s Law?
Boyle’s Law establishes an inverse relationship between the volume and pressure of a gas at constant temperature. This law enables the prediction of pressure changes resulting from volume adjustments.
How does Charles’s Law relate to temperature and volume?
Charles’s Law demonstrates a direct relationship between the temperature and volume of a gas at constant pressure. As temperature increases, volume expands, and vice versa.
What practical applications does the Combined Gas Law have?
The Combined Gas Law combines Boyle’s Law and Charles’s Law, allowing for the prediction of gas behavior under simultaneous changes in pressure, volume, and temperature. This law finds applications in fields such as scuba diving, weather forecasting, and industrial gas handling.